
Energy from Gas
There's a lot of interest in energy at the moment, with prices of gas
and oil spiralling upwards and domestic consumers probably in line for
even bigger fuel bills. Gas is a major contributor to electricity generation in
the UK; it supplied 34% of our electricity in 2004 and the proportion is
still increasing. Coal also supplied 34%, and these two fuels are the
"backbone" of our energy supply.
Natural gas is actually methane. It burns very cleanly in
our boilers and cookers, forming steam and carbon dioxide.
Methane has the formula
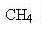
and when it burns you get carbon dioxide and water (sorry about the equations, but some of you might be interested):
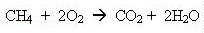
Methane adds to the carbon dioxide in the atmosphere and so
possibly contributes to global warming. But what less people know is
that when you burn methane, you will also get some acid rain.
How's this possible, bearing in mind that natural gas
contains no sulphur?
The answer is found if we look at what happens near a flame.
Our atmosphere is full of nitrogen and oxygen - about 80% and 20%.
Usually nitrogen doesn't do much - it's pretty inert - but in a flame,
strange things happen. Nitrogen combines with oxygen - like this:
nitrogen + oxygen ---> nitrogen oxide,
or, in an equation,
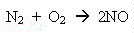
If we were to analyse the fumes in your kitchen, coming from the burners on your cooker, we'd probably find from twenty to fifty molecules in every million were nitrogen oxide.
It wouldn't matter much if yours were the only cooker. But there
are fifty million of us in the UK, most of us burn gas in our cookers
and boilers, and a third of our electricity comes from gas.
That's a lot of nitrogen oxide.
Each ton of gas forms about 50 grams,
which rapidly turns into 100 grams or so of nitric acid.
It's not long before this gets into the atmosphere. Short of
sealing up your kitchen, permanently, and blocking up your
chimney, there's nothing you can do.
It's not just gas which pollutes in this way. Whenever
a fuel is burned - any fuel - nitric acid is the result. Power stations
have strict limits on the amounts they can release (they use lime to
absorb most of it) but apart from that, it just escapes into the air. So we
get foul air in cities, erosion of buildings, damage to trees, and so on ...
We are all polluters; it's
the way we're made.
Nigel Deacon / Habitat21 website
big turbines
small turbines